University of Illinois at Urbana-Champaign
Energy Transport Research Lab
Research Statement
The Energy Transport Research Lab's research intersects the multidisciplinary fields of thermo-fluid sciences, interfacial phenomena, and energy. We aim to bring about transformational efficiency enhancements in energy (power generation, oil and gas, renewables), water, agriculture, transportation and electronics cooling by fundamentally manipulating heat-fluid-surface interactions across multiple length and time scales. The focus of our research is directed towards both: 1) fundamental research on micro/nanostructured surfaces for phase change and interfacial phenomena, and 2) applied research on devices and systems including solar thermal energy conversion, energy storage, and high power density electronics thermal management.
Nanoengineered Surfaces
Wettability
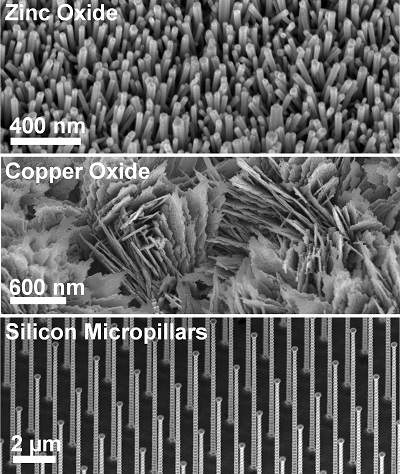
Interactions between liquids and solids are ubiquitous in our physical environment and are typically characterized by the wetting angle that a liquid droplet makes on the solid surface. While wettability on flat and homogeneous surfaces has been researched extensively, recent advances in micro-/nanofabrication and coating technologies have enabled the development of smart engineered surfaces [1, 2]. Fundamental understanding of the wetting and liquid propagation behavior on these surfaces is important for a range of applications such as microfluidics [3], thermal management [4], lab-on-a-chip devices, desalination, optical, and biological systems.
At the Energy Transport Research Lab, we are working towards developing a better understanding of the change in wettability due to surface engineering [5, 6], chemical heterogeneity [7-9], and in the presence of liquid-vapor phase change phenomena [10-13]. These studies are critical for elucidating the underlying physical mechanisms behind the other research topics being investigated in our lab. For example, hydrophilic surfaces made superhydrophilic due to structuring have resulted in enhancement of boiling and thin-film evaporation heat transfer [14]. Conversely, hydrophobic structured surfaces, i.e. superhydrophobic surfaces have recently shown a promise to push the limits of condensation heat transfer [1, 4, 15].
Evaporation and Boiling
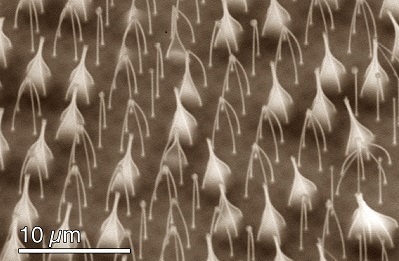
With the increase in processing speed in compact electronic devices, passive heat transfer cooling technologies with the ability cool heat fluxes of up to 1000 W/cm2 are highly desired. Conventional air cooling strategies are insufficient at these large heat fluxes. As a result, novel thermal management solutions such as thin-film evaporation that utilize the latent heat of vaporization of a fluid are needed [16]. The high latent heat of vaporization associated with typical liquid-vapor phase change phenomena allows significant heat transfer with little temperature rise [17].
At the Energy Transport Research Lab, we are using a combination of state-of-the-art silicon fabrication techniques with highly scalable metal oxidation and etching to fabricate micro/nanostructured surfaces to implement thin-film evaporation [5], pool boiling [14], and flow boiling. The structures improve liquid spreading by generating capillary pressure in addition to increasing the thin-film region where the majority of the evaporation occurs. Moreover, the liquid-film thickness and the associated thermal resistance is minimum in the thin-film region making evaporation an attractive choice for dissipating high heat fluxes. However, one limitation in using microstructured surfaces for thin-film evaporation is dry out which occurs when the generated capillary pressure cannot transport enough liquid to sustain evaporation. To overcome the capillary pressure budget limitation, we use wicks and surface structures to enhance liquid spreading as well as to achieve high heat fluxes with high heat transfer coefficients [14]. Nanostructure (first level porosity) is used to generate higher capillary pressure that assists liquid spreading while larger microchannels (second level porosity) are used to reduce the overall viscous loss by providing a less-viscous bypass path for fluid flow.
Condensation

Condensation is a phase change phenomenon often encountered in nature, as well as in industry for applications including power generation, thermal management, desalination, and building environmental control. For the past eight decades, researchers have focused on creating surfaces allowing condensed droplets to be easily removed by gravity for enhanced heat transfer performance [1]. Recent advancements in nanofabrication have enabled increased control of surface structuring for the development of superhydrophobic surfaces with even higher droplet mobility and, in some cases, coalescence-induced droplet jumping [18-20].
At the Energy Transport Research Lab, we are theoretically [19, 20], numerically [18, 21, 22], and experimentally [23-28] studying superhydrophobic and oleophobic [12, 29, 30] surfaces for enhanced condensation heat transfer for water, petrochemical fluid, and refrigerant condensation.
In addition, we study the fabrication, characterization [6], wettability, and interfacial dynamics of superhydrophobic materials during condensation to examine the role of surface structure on emerging droplet morphology [31, 32], nucleation density [4, 33-35], droplet growth rate [8, 11, 13, 36], and departure characteristics. Furthermore, we seek to develop scalable fabrication techniques for creating superhydrophobic surfaces with experimentally demonstrated heat transfer enhancement [23, 37-39]..
Lastly, we focus on studying the fundamental degradation mechanisms of hydrophobic and superhydrophobic surfaces [24, 40-42] with significant collaboration with industry to develop durable and robust coatings that can last for multiple years without re-application [43, 44].
Freezing
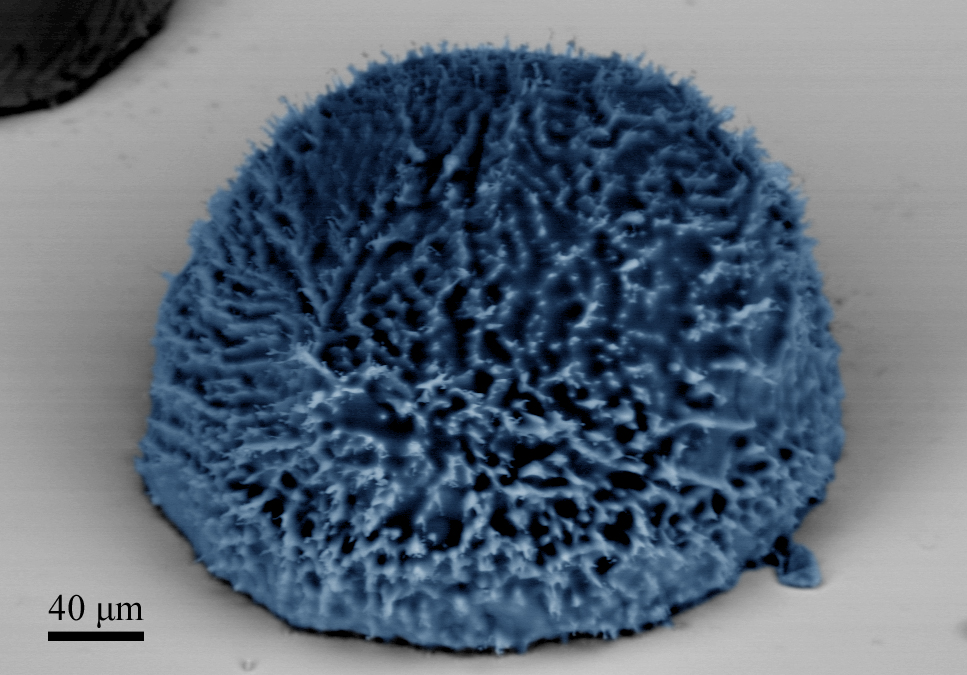
Frost and ice has long been a problem in the eyes of human society. The effects of frosting and icing can have dramatic consequences: downed power lines, damaged crops, stalled aircraft, as well as decreased performance of ships, wind turbines, and heating ventilation and air conditioning (HVAC) systems [45]. The buildup of frost on HVAC components results in a continuous decrease in cooling capacity, increased pressure drop, and therefore increased pumping costs. Currently used active chemical, thermal, and mechanical methods of ice and frost removal are costly. Therefore, the development of passive methods and ultra-efficient active methods to prevent frost and ice accumulation is desirable.
The current approach to fabricate frost reducing surfaces focuses on the development of rough hydrophobic surfaces to increase the energy barrier for ice nucleation [46], and to further reduce both contact angle hysteresis, and the ice adhesion strength. Using this approach, many recent studies have shown superhydrophobic surfaces to be successful in preventing ice buildup for individual droplets being deposited on the surface or impacting the surface at some prescribed velocity. However the typical working conditions encountered in industrial HVAC applications are better represented by condensation frosting, where the surrounding moist air first condenses on the surface to form liquid microdroplets that grow via coalescence, nucleate ice and grow in the form of frost. When condensation frosting occurs on a superhydrophobic surface, the formation of highly mobile ‘Cassie’ droplets cannot be ensured, and the nucleating ice can impale the structure and result in increased ice-adhesion. Hence, the early and energy-efficient removal of condensed microdroplets from the supercooled surface is of high priority.
At the Energy Transport Research Lab, we are currently studying a new approach to creating frost-reducing surfaces consisting of suitably designed, and highly scalable metallic superhydrophobic surfaces [47]. The key innovation in the proposed concept is the incorporation of the novel superhydrophobic nanostructures on the frosting surfaces (i.e. evaporator fins). Accordingly, the new mode of jumping-droplet-condensation, which has been previously demonstrated [37], can be harnessed to achieve an order of magnitude increase in heat transfer and hence condensed water removal rate (prior to frosting), compared to that of smooth hydrophilic metal surfaces [10].
In addition to delayed condensation frosting, we are also investigating defrosting and deicing approaches using pulsed electro-thermal heating [48], and wetting heterogeneity (biphilic surfaces) to enable enhanced frost and ice mobility.
Fouling and Corrosion
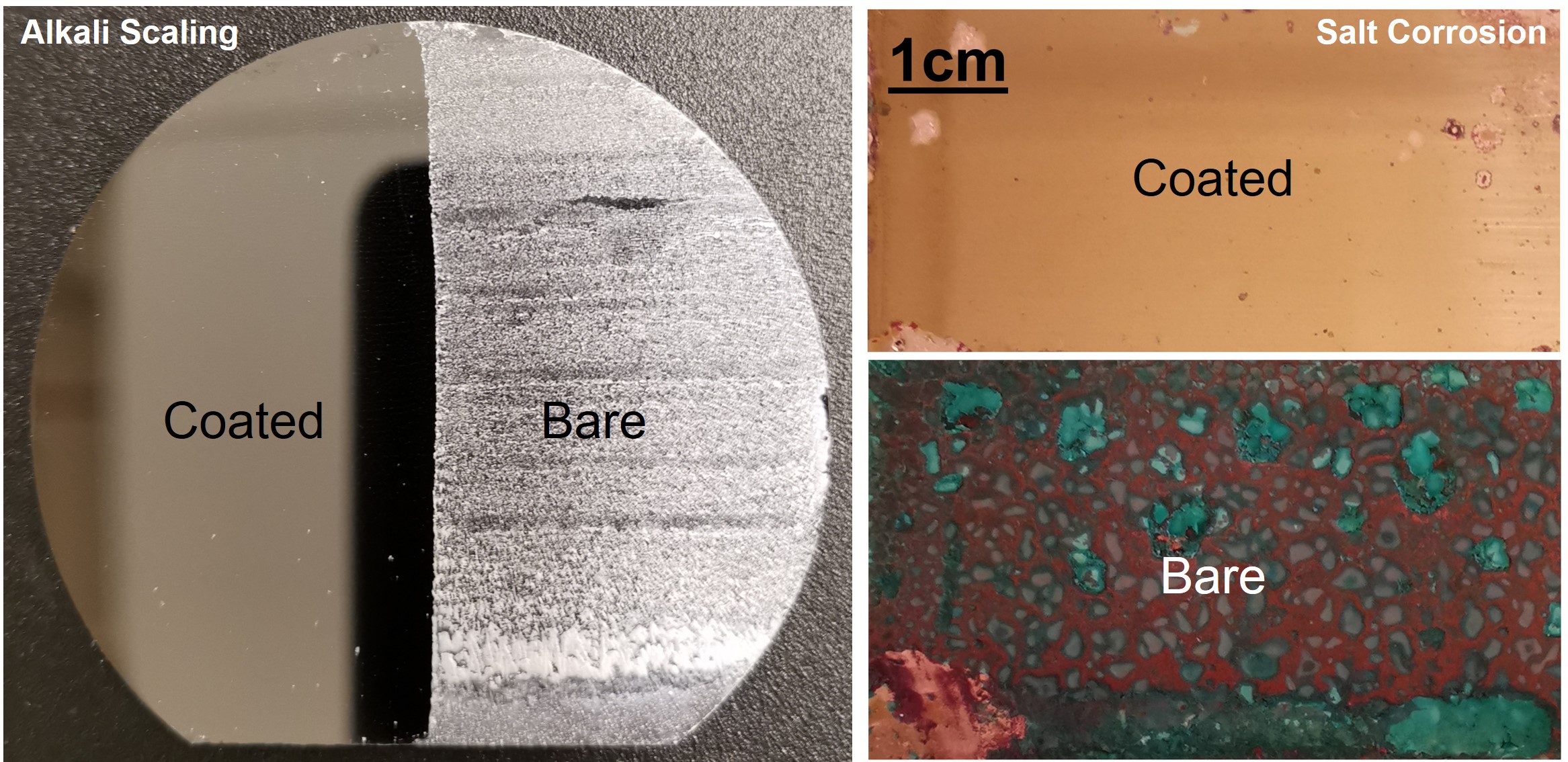
Fouling is a major unresolved problem in a variety of industries including water treatment and power generation. In heat exchangers, accumulation of salt scale on surfaces can lead to decreased heat transfer, increased corrosion, and increased pressure drop, resulting in higher operational costs and shortened equipment lifetime. The added operational costs for active scale removal account for 0.25% of the gross domestic product and 2.5% of the CO2 emissions in industrialized nations.In the past few decades, an emphasis has been placed on developing techniques to enable scale mitigation. These efforts have mostly focused on improving the efficiency of mechanical and chemical removal techniques. Recent methods have proposed altering the surface structure and chemistry to control the heterogeneous nucleation rate of scale formation. According to classical nucleation theory, lowering the surface energy of the substrate increases the energy barrier for heterogeneous nucleation and thus leads to delayed salt scaling. Furthermore, surfaces that are rendered ultra-smooth or atomically smooth provide fewer sites for heterogeneous nucleation, leading to reduced scaling rates.
A promising method to achieve simultaneous reduction in surface energy and roughness is through the fabrication of slippery liquid infused porous surfaces (SLIPSs) or lubricant infused surfaces (LISs). The main drawback of SLIPSs and LISs is their propensity to degrade over time due to lubricant drainage, especially in applications where flow is present.
In order to mitigate lubricant drainage, at the Energy Transport Research Lab, we are developing slippery omniphobic covalently attached liquids (SOCAL) as a means to achieve durable antiscaling function on metallic substrates [49]. The SOCAL coating has great potential for antiscaling performance due to its ultra-low surface energy and high topographical homogeneity. We have developed and demonstrated multiple methods to deposit SOCAL coatings on arbitrary surfaces through electrophoretic deposition (EPD) of SiO2 as an intermediary layer between the arbitrarily shaped substrate and the coating.
In addition to fouling, corrosion is a major problem for a variety of industries leading to metallic surface degradation. The economic impact of corrosion is estimated to be between 2 and 5% of the global gross national product. In thermal systems utilizing heat exchangers, corrosion inside of tubes and channels decreases heat transfer, increase nucleation site density for heterogeneous scale formation, acts as a fouling product (corrosion fouling), and increases pressure drop, resulting in lower efficiency and eventually reducing the lifespan of the system. To combat corrosion, corrosion prevention coatings have been developed in the past.
To enable greater scalability to a variety of systems, as well as to reduce cost and increase ease of implementation, great attention has been given to sol-gel coatings of SiO2. Sol-gel SiO2 possesses many advantages, such as eco-friendly chemistry, great stability, and deposition capability at relatively low temperatures. The low temperature and atmospheric pressure sol-gel process makes it particularly advantageous to chemical vapor deposition of SiO2 which typically require well controlled environmental conditions with temperatures approaching 350C, and can affect the metallurgy of the substrate.
At the Energy Transport Research Lab, we are currently studying both corrosion fundamentals and mechanisms on a variety of metals as well as coatings. Of particular interest to us are highly scalable sol-gel SiO2 approaches that can be applied to arbitrary metals at room temperature and pressure.
Droplets

Droplet transport on and shedding from a surface is vital to the cleaning of plants leaves and insect wings [50], anti-bacterial function of animal skins, and spreading of basidiomycete ballistospores. In the industrial world, rapid shedding of droplets from solid surfaces has become the primary bottleneck hindering the enhancement of a plethora of applications ranging from self-cleaning, anti-fogging, anti-frosting, water harvesting, to condensation heat transfer. In an effort to enhance droplet shedding, various approaches have been proposed to realize passive or active droplet transport enabled by external electric fields [51-54], vapor cushions, and capillary and gravitational forces. Among them, droplet self-transport induced by surface chemistry and/or structural properties including wettability gradients, charge density gradients, structure variations, and diverging tracks have received increasing attention due to their passive nature.
At the Energy Transport Research Lab, we are studying droplet-droplet and droplet-surface interactions with a focus on fluid flow hydrodynamics at micrometric length scales with high temporal resolution [31, 32]. The fundamental understanding unlocked form the study of these interaction mechanisms has enabled us us to develop key innovations for many applications and devices [55-57].
Metrology
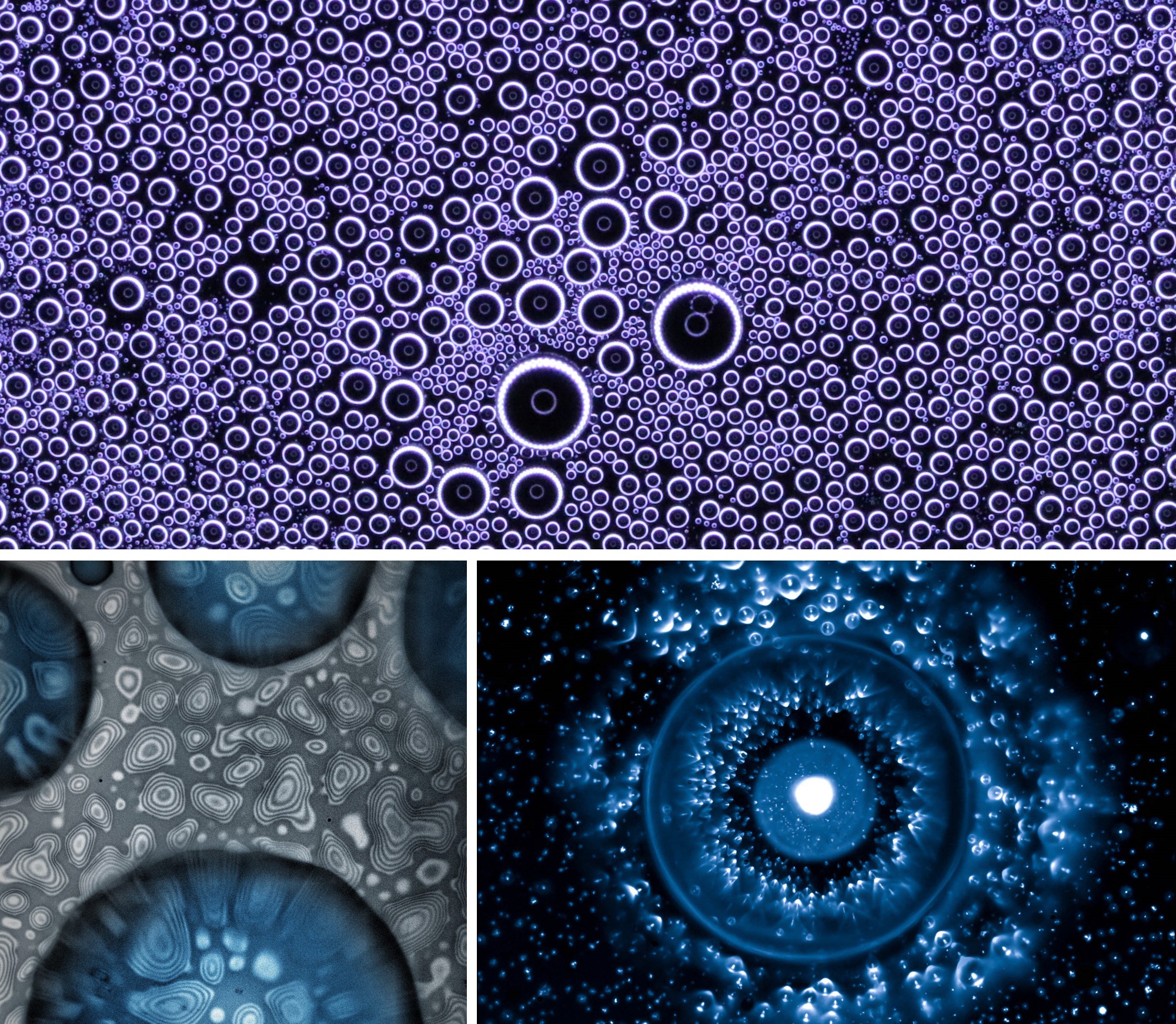
The study of micro/nanoscale phase change phenomena as well as wetting interactions between droplets, bulk liquids, and solids is limited by the lack of observation techniques capable of resolving the required spatial and temporal scales. In the Energy Transport Research Laboratory, we are addressing these challenges through the simultaneous extraction of mechanical and chemical information using novel sensing platforms developed in our lab [58-60] such as Focal Plane Shift Imaging (FPSI) which is able to observe 3D dynamic wetting phenomena at the microscale for a multitude of processes [61]. Using FPSI, we have answered fundamental questions persisting in the coalescence induced droplet jumping literature, such as understanding of droplet directionality and effect of multi-droplet coalescence on departure velocity. The FPSI technique provides a powerful imaging platform for the study of dynamic processes such as sliding, coalescence, or impact.
In addition to FPSI, we have developed an evaporation metrology technique to study Temporally and Spatially Steady Evaporation [62]. Traditionally, researchers have studied evaporation by observing the change in the droplet size in a given time interval. However, the transient nature coupled with the significant mass-transfer-governed gas dynamics occurring at the droplet three-phase contact line makes the classical method crude. Furthermore, the intricate balance played by the internal and external flows, evaporation kinetics, thermocapillarity, binary-mixture dynamics, curvature, and moving contact lines makes the decoupling of these processes impossible with classical transient methods. To cope with these difficulties, we have recently developed a method to measure the rate of evaporation of spatially and temporally steady droplets using a frequency-modulated piezoelectric dispenser to feed microscale droplets to a larger evaporating droplet. Our technique provides an experimental platform to enable the decoupling of the complex physics governing the ubiquitous droplet evaporation process.
Laser-ablation electrospray ionization (LAESI) imaging mass spectrometry (IMS) is an emerging bioanalytical tool for direct imaging and analysis of biological tissues. When combined with optical microscopy, the investigation of biological samples by LAESI allows for spatially resolved compositional analysis. We have demonstrated the applicability of LAESI-IMS for the chemical analysis of thin, desiccated biological samples, specifically Neotibicen pruinosus cicada wings [63]. Various putative chemical identifications were made indicating the presence of hydrocarbons, lipids/esters, amines/amides, and sulfonated/phosphorylated compounds. With the spatial resolution capability, surprising chemical distribution patterns were observed across the cicada wing, which assisted in correlating trends in surface properties with chemical distribution from a previous study of ours. Our findings demonstrate LAESI-IMS as a tool for the acquisition of spatially resolved chemical information from fragile biological samples.
Energy
Solar
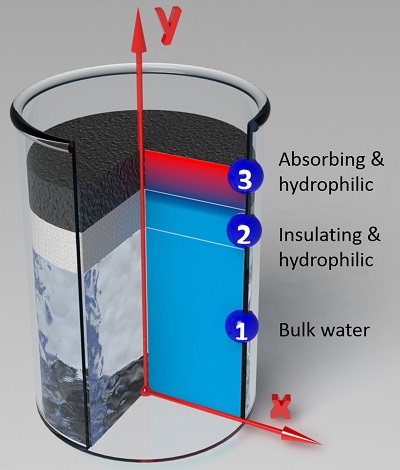
Solar irradiation is a promising source of renewable energy, as the hourly incident solar flux on the surface of the earth is greater than annual global energy consumption [64]. Efficient harvesting of solar energy for steam generation is a key factor for a broad range of applications, from large-scale power generation, absorption chillers and desalination systems to compact applications such as water purification for drinking, sterilization and hygiene systems in remote areas where the only abundant energy source is the sun. Current methods of generating steam using solar energy rely on a surface or cavity to absorb the solar radiation, and transferring heat to the bulk liquid directly or via an intermediate carrier fluid, which require high optical concentration and suffer from high optical loss and surface heat loss, or vacuum to reduce convective heat loss under moderate optical concentration. The steam thus generated is usually in thermal equilibrium with the bulk liquid. Nanofluids—fluids seeded with nanoparticles—as another alternative have been studied as volumetric absorbers, potentially minimizing the surface energy loss by uniform temperature in the fluid. Local generation of steam in a cold bulk liquid can be achieved through high concentrations or illumination of nanofluids by electromagnetic waves (generally, lasers) with high power intensity. However, the high costs and optical concentrations limit the utilization of these approaches in stand-alone compact solar systems.
At the Energy Transport Research Lab, we are working on a new approach and corresponding material structure that localizes the solar energy where evaporation occurs and minimizes the heat losses leading to enhanced solar thermal efficiency at low optical concentration in open air while generating steam [64]. Under solar illumination, the developed structure forms a hot spot internally where evaporation occurs [65, 66]. The fluid wicks to the hot spot, evaporates and forms vapor which leaves the structure. This structure needs to have four main characteristics: high absorption in the solar spectrum, low thermal conductivity to suppress thermal conduction away from the hot internal region, hydrophilic surfaces to leverage capillary forces and promote fluid flow to the hot region, and interconnected pores for fluid flow to and from the structure.
Energy Harvesting
Energy harvesting or “energy scavenging” is the conversion of ambient energy present in the environment into electrical energy. Typically, energy harvesting involves the conversion of small amounts of ambient energy to power small (<1 cm), low-power (<1 µW) electronic devices. In addition to being pollution free, the harvested energy is usually derived from waste energy streams that are otherwise not harnessed for useful work. Energy harvesting has therefore attracted much interest because of its potential use as a power supply in applications such as low-power wireless sensor networks and electronic systems.
At the Energy Transport Research Lab, we are interested in jumping-droplet- based energy harvesting with nanoengineered superhydrophobic surfaces. Recent studies have shown that when small water droplets (<10–100 µm) merge on superhydrophobic nanostructured surfaces, droplets can spontaneously eject and charge via the release of excess surface energy irrespective of gravity [67]. We are interested in taking advantage of this unique droplet-charging phenomenon to study jumping-droplet energy harvesting, where the charged droplets jump between superhydrophobic and hydrophilic surfaces to create an electrostatic potential and generate power by direct condensation of atmospheric moisture.
Thermal Management
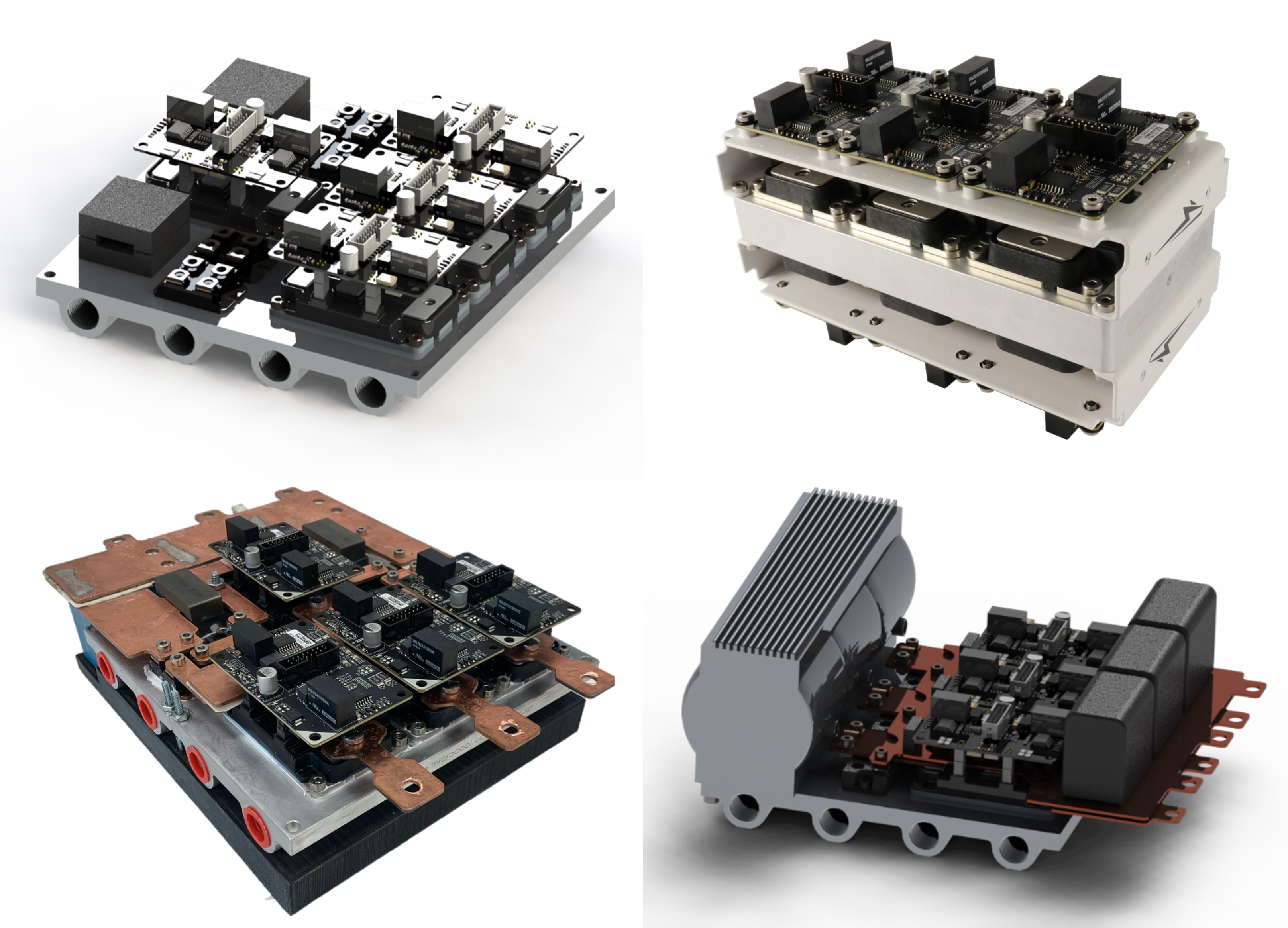
Devices capable of actively controlling heat flow have been desired by the thermal management community for decades [68, 69]. The need for thermal control has become particularly urgent with power densification resulting in devices with localized heat fluxes as high as 1 kW/cm2. At the Energy Transport Research Lab, we recently demonstrated a millimeter-scale thermal switch with a switching ratio >70, at heat fluxes near 10 W/cm2 [70, 71]. Furthermore, we have extended the fundamental phase change heat transfer efforts to demonstrate jumping-droplet condensation as a method to achieve active hot spot cooling in electronic devices [7, 17]. Demonstration of dynamic switching of the electric field for a two-GaN system revealed the potential for active cooling of mobile hot spots.
In addition to thermal switching and routing, thermal management of electronics' active and passive components as well a assemblies (images above) remains an issue for many vital energy applications [72]. Thermal management is a critical bottleneck to increasing computational performance of modern data centers in terms of density (Gflops/m3) and efficiency (Gflops/W). On average, approximately 20-30% of the energy demand from a data center is utilized to reject heat. Furthermore, server packaging density is limited by the spatial requirements of air or single-phase liquid architectures. Two shortcoming of modern air- and liquid-cooled thermal management schemes are the thermal interface material (TIM), which provides mechanical compliance and electrical isolation at the cost of poor heat transfer. Due to the poor heat transfer of the TIM, larger heat sinks or faster fluid flow rates are required to reject the heat. While most high heat flux and TIM-less methods such as oil spray and jet impingement, require large capital investment for pumps, integration, and safety, we seek to develop low-cost, scalable methods for coating server hardware so that it can be cooled efficiently with direct immersion in water and push the bounds of computational density offered by promising state of the art (SOA) approaches. We have recently demonstrated phase-change cooling with water greater than 500 W/cm2 while maintaining chip junction temperatures within safe temperature limits [73], a dramatic improvement over the 250 W/cm2 capable with aggressive industrial approaches today. By facilitating both faster clock speeds and closer packing of components than can be achieved with other SOA cooling schemes, we seek to demonstrate a 10x improvement in computational density. Thus, direct immersion in water has the potential to facilitate the deployment of data centers with next generation GPUs and CPUs for machine learning and big data analytics.
References
- Enright, R., et al., Dropwise Condensation on Micro- and Nanostructured Surfaces. Nanoscale and Microscale Thermophysical Engineering, 2014. 18(3): p. 223-250.
- Hoque, M.J., et al., High-Throughput Stamping of Hybrid Functional Surfaces. Langmuir, 2020. 36(21): p. 5730-5744.
- Oh, J., et al., Cicada-inspired self-cleaning superhydrophobic surfaces. Journal of Heat Transfer-Transactions of the Asme, 2019. 141(10).
- Miljkovic, N. and E.N. Wang, Condensation heat transfer on superhydrophobic surfaces. Mrs Bulletin, 2013. 38(5): p. 397-406.
- Miljkovic, N., et al., Liquid Evaporation on Superhydrophobic and Superhydrophilic Nanostructured Surfaces. Journal of Heat Transfer-Transactions of the Asme, 2011. 133(8).
- Enright, R., et al., Condensation on Superhydrophobic Surfaces: The Role of Local Energy Barriers and Structure Length Scale. Langmuir, 2012. 28(40): p. 14424-14432.
- Foulkes, T., et al., Self-assembled liquid bridge confined boiling on nanoengineered surfaces. International Journal of Heat and Mass Transfer, 2019. 133: p. 1154-1164.
- Hoque, M.J., et al., Visualization of Droplet Nucleation on Patterned Hybrid Surfaces. Journal of Heat Transfer-Transactions of the Asme, 2019. 141(10).
- Preston, D.J., et al., Effect of hydrocarbon adsorption on the wettability of rare earth oxide ceramics. Applied Physics Letters, 2014. 105(1).
- Boyina, K., et al., Condensation frosting on meter-scale superhydrophobic and superhydrophilic heat exchangers. International Journal of Heat and Mass Transfer, 2019. 145(118694).
- Cha, H., et al., Dropwise Condensation on Solid Hydrophilic Surfaces. Science Advances, 2019. in press.
- Sett, S., et al., Stable Dropwise Condensation of Ethanol and Hexane on Rationally Designed Ultrascalable Nanostructured Lubricant-Infused Surfaces. Nano Letters, 2019. 19(8): p. 5287-5296.
- Yan, X., et al., Hierarchical Condensation. Acs Nano, 2019. 13(7): p. 8169-8184.
- Li, J.Q., et al., Ultrascalable Three-Tier Hierarchical Nanoengineered Surfaces for Optimized Boiling. Acs Nano, 2019. 13(12): p. 14080-14093.
- Bhatia, B., et al., Nanoengineered Surfaces for Thermal Energy Conversion. 15th International Conference on Micro and Nanotechnology for Power Generation and Energy Conversion Applications (Powermems 2015), 2015. 660.
- Voglar, J., et al., Analysis of heater-wall temperature distributions during the saturated pool boiling of water. Experimental Thermal and Fluid Science, 2019. 102: p. 205-214.
- Oh, J., et al., Jumping-droplet electronics hot-spot cooling. Applied Physics Letters, 2017. 110(12).
- Enright, R., et al., How Coalescing Droplets Jump. Acs Nano, 2014. 8(10): p. 10352-10362.
- Birbarah, P. and N. Miljkovic, Internal convective jumping-droplet condensation in tubes. International Journal of Heat and Mass Transfer, 2017. 114: p. 1025-1036.
- Birbarah, P. and N. Miljkovic, External convective jumping-droplet condensation on a flat plate. International Journal of Heat and Mass Transfer, 2017. 107: p. 74-88.
- Birbarah, P., S. Chavan, and N. Miljkovic, Numerical Simulation of Jumping Droplet Condensation. Langmuir, 2019. 35(32): p. 10309-10321.
- Miljkovic, N., R. Enright, and E.N. Wang, Modeling and Optimization of Superhydrophobic Condensation. Journal of Heat Transfer-Transactions of the Asme, 2013. 135(11).
- Razayi, S.M.R., et al., Superhydrophobic Surfaces Made from Naturally Derived Hydrophobic Materials. Acs Sustainable Chemistry & Engineering, 2017. 5(12): p. 11362-11370.
- Sett, S., et al., Lubricant-Infused Surfaces for Low-Surface-Tension Fluids: Promise versus Reality. Acs Applied Materials & Interfaces, 2017. 9(41): p. 36400-36408.
- Shahriari, A., et al., Electric Field-Based Control and Enhancement of Boiling and Condensation. Nanoscale and Microscale Thermophysical Engineering, 2017. 21(2): p. 102-121.
- Weisensee, P.B., et al., Droplet impact on vibrating superhydrophobic surfaces. Physical Review Fluids, 2017. 2(10).
- Oh, J., et al., Thin Film Condensation on Nanostructured Surfaces. Advanced Functional Materials, 2018. 28(16).
- Wu, A. and N. Miljkovic, Droplet Cloaking Imaging and Characterization. Journal of Heat Transfer-Transactions of the Asme, 2018. 140(3).
- Ge, Q.Y., et al., Condensation of Satellite Droplets on Lubricant-Cloaked Droplets. Acs Applied Materials & Interfaces, 2020. 12(19): p. 22246-22255.
- Ge, Q.Y., et al., Water condensation on ionic liquid infused nanostructured surfaces. Abstracts of Papers of the American Chemical Society, 2019. 258.
- Miljkovic, N., R. Enright, and E.N. Wang, Effect of Droplet Morphology on Growth Dynamics and Heat Transfer during Condensation on Superhydrophobic Nanostructured Surfaces. Acs Nano, 2012. 6(2): p. 1776-1785.
- Yan, X., et al., Droplet Jumping: Effects of Droplet Size, Surface Structure, Pinning, and Liquid Properties. Acs Nano, 2019. 13(2): p. 1309-1323.
- Miljkovic, N., et al., Jumping Droplet Dynamics on Scalable Nanostructured Superhydrophobic Surfaces. Journal of Heat Transfer-Transactions of the Asme, 2013. 135(8).
- Miljkovic, N., et al., Condensation on Hydrophilic, Hydrophobic, Nanostructured Superhydrophobic and Oil-Infused Surfaces. Journal of Heat Transfer-Transactions of the Asme, 2013. 135(8).
- Xiao, R., et al., Immersion Condensation on Oil-Infused Heterogeneous Surfaces for Enhanced Heat Transfer. Scientific Reports, 2013. 3.
- Yan, X., et al., Atmosphere-Mediated Superhydrophobicity of Rationally Designed Micro/Nanostructured Surfaces. Acs Nano, 2019. 13(4): p. 4160-4173.
- Miljkovic, N., et al., Jumping-Droplet-Enhanced Condensation on Scalable Superhydrophobic Nanostructured Surfaces. Nano Letters, 2013. 13(1): p. 179-187.
- Preston, D.J., et al., Scalable Graphene Coatings for Enhanced Condensation Heat Transfer. Nano Letters, 2015. 15(5): p. 2902-2909.
- Razavi, S.M.R., et al., Environment-Friendly Antibiofouling Superhydrophobic Coatings. Acs Sustainable Chemistry & Engineering, 2019. 7(17): p. 14509-14520.
- Cha, H.Y., et al., Nanoscale-Agglomerate-Mediated Heterogeneous Nucleation. Nano Letters, 2017. 17(12): p. 7544-7551.
- Bakir, M., et al., Effects of environmental aging on physical properties of aromatic thermosetting copolyester matrix neat and nanocomposite foams. Polymer Degradation and Stability, 2018. 147: p. 49-56.
- Ma, J.C., et al., Condensation Induced Delamination of Nanoscale Hydrophobic Films. Advanced Functional Materials, 2019. 29(43).
- Chang, H.C., et al., Composite Structured Surfaces for Durable Dropwise Condensation. International Journal of Heat and Mass Transfer, 2020. 156.
- Ma, J.C., et al., Recent developments, challenges, and pathways to stable dropwise condensation: A perspective. Applied Physics Letters, 2020. 116(26).
- Chavan, S., et al., Bulk water freezing dynamics on superhydrophobic surfaces. Applied Physics Letters, 2017. 110(4).
- Miljkovic, N., R. Enright, and E.N. Wang, Liquid Freezing Dynamics on Hydrophobic and Superhydrophobic Surfaces. Journal of Heat Transfer-Transactions of the Asme, 2012. 134(8).
- Chavan, S., et al., Effect of Latent Heat Released by Freezing Droplets during Frost Wave Propagation. Langmuir, 2018. 34(22): p. 6636-6644.
- Chavan, S., et al., Pulse interfacial defrosting. Applied Physics Letters, 2019. 115(7).
- Zhao, H.Y., et al., Extreme Antiscaling Performance of Slippery Omniphobic Covalently Attached Liquids. Acs Applied Materials & Interfaces, 2020. 12(10): p. 12054-12067.
- Oh, J., et al., Exploring the Role of Habitat on the Wettability of Cicada Wings. Acs Applied Materials & Interfaces, 2017. 9(32): p. 27173-27184.
- Miljkovic, N., et al., Electric-Field-Enhanced Condensation on Superhydrophobic Nanostructured Surfaces. Acs Nano, 2013. 7(12): p. 11043-11054.
- Miljkovic, N., et al., Electrostatic charging of jumping droplets. Nature Communications, 2013. 4.
- Preston, D.J., et al., Jumping Droplet Electrostatic Charging and Effect on Vapor Drag. Journal of Heat Transfer-Transactions of the Asme, 2014. 136(8).
- Cavalli, A., et al., Electrically induced drop detachment and ejection. Physics of Fluids, 2016. 28(2).
- Foulkes, T., et al., Jumping droplets electronics cooling: Promise versus reality. Applied Physics Letters, 2020. 116(20).
- Foulkes, T., et al., Fundamental limits of jumping droplet heat transfer. Applied Physics Letters, 2020. 116(9).
- Rajagopal, M.C., et al., Materials-to-device design of hybrid metal-polymer heat exchanger tubes for low temperature waste heat recovery. International Journal of Heat and Mass Transfer, 2019. 143.
- Ma, J.C., D.G. Cahill, and N. Miljkovic, Condensation Induced Blistering as a Measurement Technique for the Adhesion Energy of Nanoscale Polymer Films. Nano Letters, 2020. 20(5): p. 3918-3924.
- Roman-Kustas, J., et al., Molecular and Topographical Organization: Influence on Cicada Wing Wettability and Bactericidal Properties. Advanced Materials Interfaces, 2020. 7(10).
- Yan, X., et al., "Swimming Jellyfish": Visualizing Jet-Like Internal Flow in Coalescing Droplets. Journal of Heat Transfer-Transactions of the Asme, 2019. 141(10).
- Cha, H., et al., Focal Plane Shift Imaging for the Analysis of Multi-Droplet Jumping. Journal of Heat Transfer-Transactions of the Asme, 2017. 139(2).
- Gunay, A.A., et al., Steady Method for the Analysis of Evaporation Dynamics. Langmuir, 2017. 33(43): p. 12007-12015.
- Roman, J.K., et al., Spatially resolved chemical analysis of cicada wings using laser-ablation electrospray ionization (LAESI) imaging mass spectrometry (IMS). Analytical and Bioanalytical Chemistry, 2018. 410(7): p. 1911-1921.
- Gunay, A.A., et al., Optically Transparent Thermally Insulating Silica Aerogels for Solar Thermal Insulation. Acs Applied Materials & Interfaces, 2018. 10(15): p. 12603-12611.
- Ghasemi, H., et al., Solar steam generation by heat localization. Nature Communications, 2014. 5.
- Ni, G., et al., Volumetric solar heating of nanofluids for direct vapor generation. Nano Energy, 2015. 17: p. 290-301.
- Miljkovic, N., et al., Jumping-droplet electrostatic energy harvesting. Applied Physics Letters, 2014. 105(1).
- Kwon, B., et al., Air Jet Impingement Cooling of Electronic Devices Using Additively Manufactured Nozzles. Ieee Transactions on Components Packaging and Manufacturing Technology, 2020. 10(2): p. 220-229.
- Moon, H., N. Miljkovic, and K.W. P., High power density thermal energy storage using additively manufactured heat exchangers and phase change material. International Journal of Heat and Mass Transfer, 2020. 153(119591).
- Yang, T.Y., et al., Millimeter-scale liquid metal droplet thermal switch. Applied Physics Letters, 2018. 112(6).
- Yang, T.Y., et al., An Integrated Liquid Metal Thermal Switch for Active Thermal Management of Electronics. Ieee Transactions on Components Packaging and Manufacturing Technology, 2019. 9(12): p. 2341-2351.
- Barth, C., et al., Experimental Evaluation of a 1 kW, Single-Phase, 3-Level Gallium Nitride Inverter in hxtreme Cold Environment. 2017 Thirty Second Annual Ieee Applied Power Electronics Conference and Exposition (APEC), 2017: p. 717-723.
- Birbarah, P., et al., Water immersion cooling of high power density electronics. International Journal of Heat and Mass Transfer, 2020. 147.
Facilities and Equipment
The Energy Transport Research Laboratory has a lab area of 3000 ft 2 with access to a shared laminar fume hood room with 2 laminar fume hoods. The laboratory features state-of-the-art control, optical, diagnostics and thermal measurement capabilities that are regularly used and updated.
For a full list of both custom equipment as well as characterization equipment in the laboratory, please see the following PDF document with detailed individual descriptions, purpose, and specifications included.
In addition to our own laboratory facilities linked above, a partial list of our facilities can be found here with a few videos to demonstrate the operation. Furthermore, we are part of the POETS center, having access to additional world-class electro-thermal testbed facilities on the Illinois campus. For a list of POETS facilities, see this link.
In addition, structured surface characterization will be performed at the Materials Research Laboratory (MRL), Micro-Nano-Mechanical Systems Cleanroom (MNMS), and the Micro-Nano Technologies Laboratory (MNTL). All three facilities are interdepartmental micro/nanofabrication and characterization facilities on the University of Illinois campus.