University of Illinois at Urbana-Champaign
Energy Transport Research Lab
Image Gallery
ETRL Gallery
Outreach Gallery
Video Gallery
Droplet Impact on Elastic Superhydrophobic Surfaces
High speed videos of droplet impact on elastic or flexible superhydrophobic surfaces. The surface elasticity causes droplet 'springboarding' and lower droplet contact times.
Charged Droplets
Nenad discusses his previous work on condensation, nanodroplet formation, and new nanopatterned surfaces that could boost the efficiency of power plants and desalination systems.
Charged Droplets
Droplets falling from a superhydrophobic surface are drawn toward an electrically charged wire, bottom center, demonstrating that they carry an electric charge.
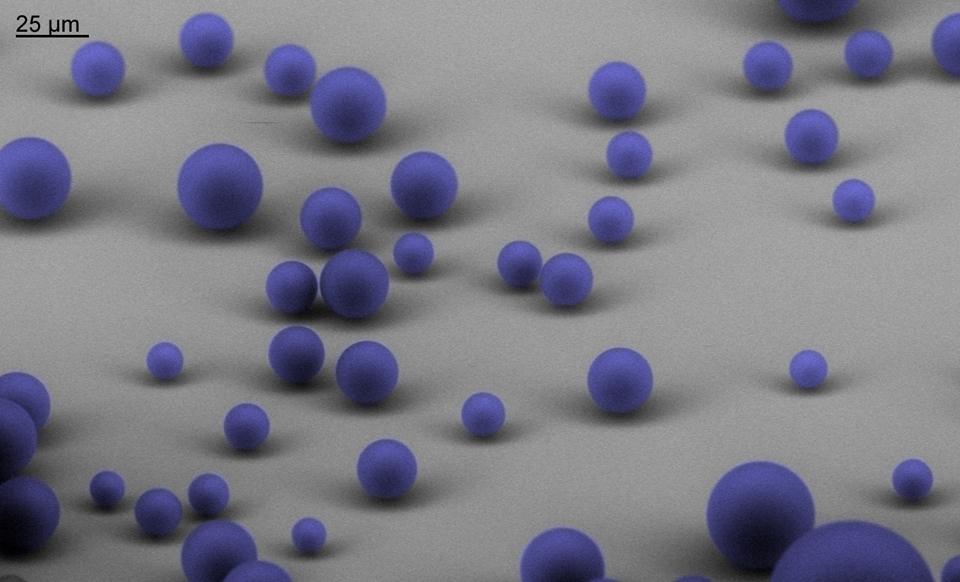
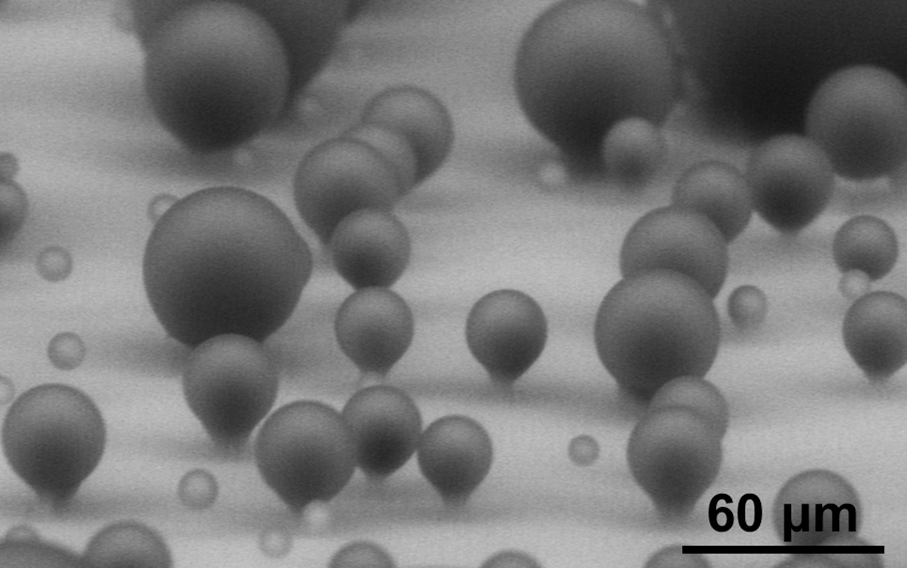
Dynamics of Coalescence-Induced Jumping Water Droplets
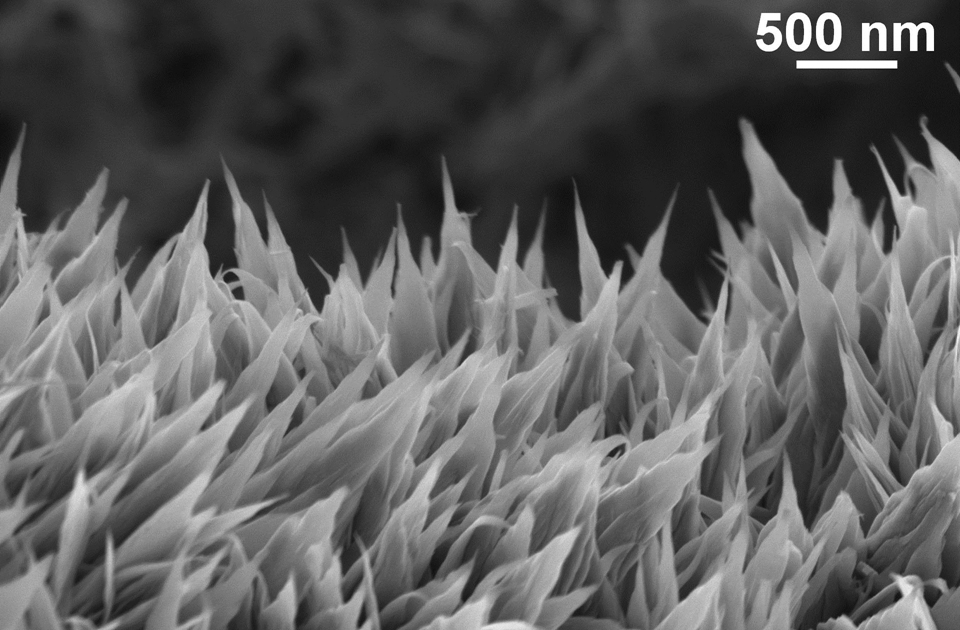
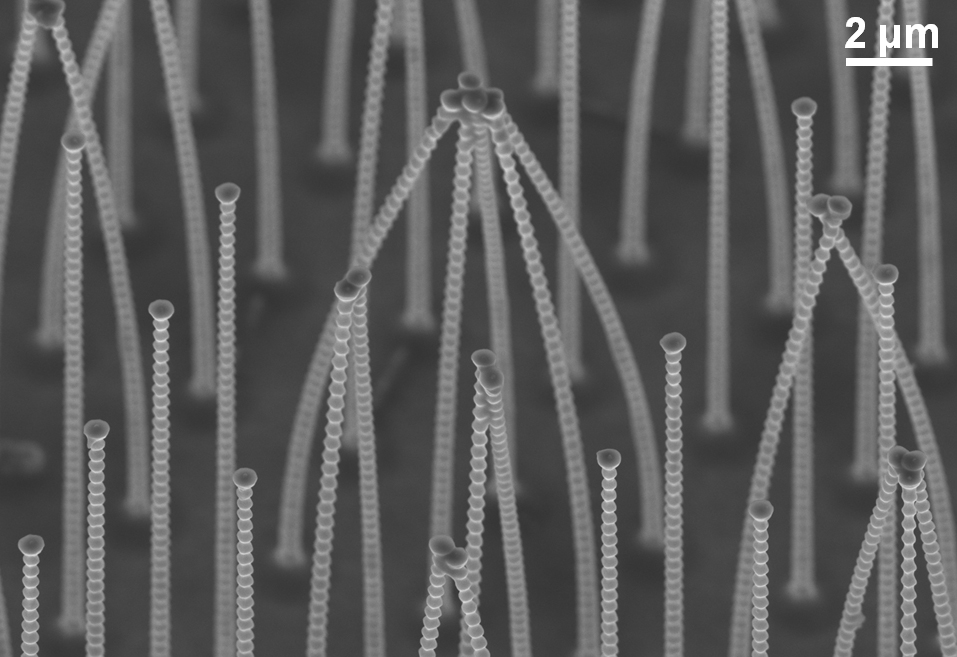
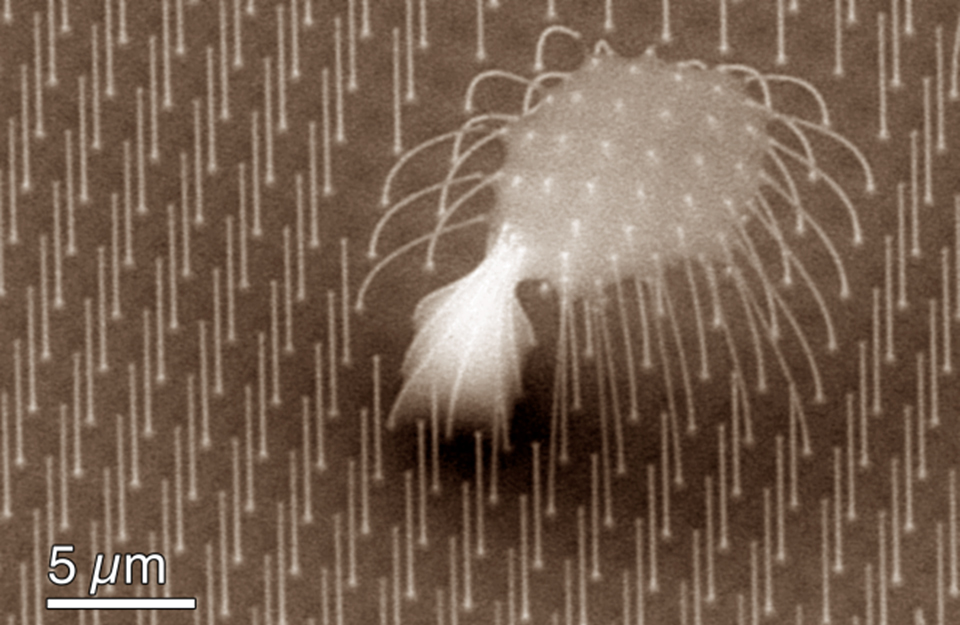
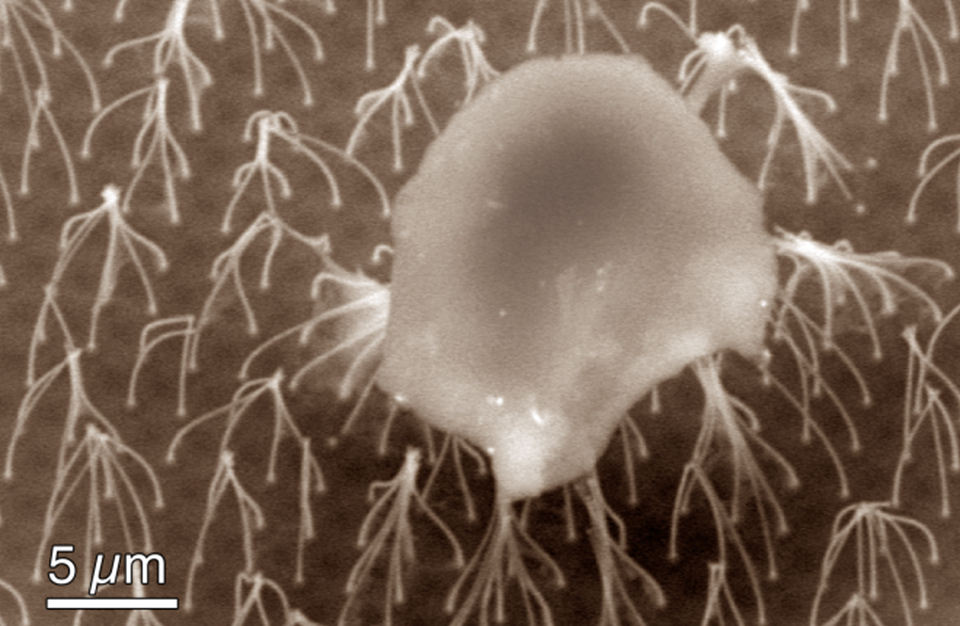
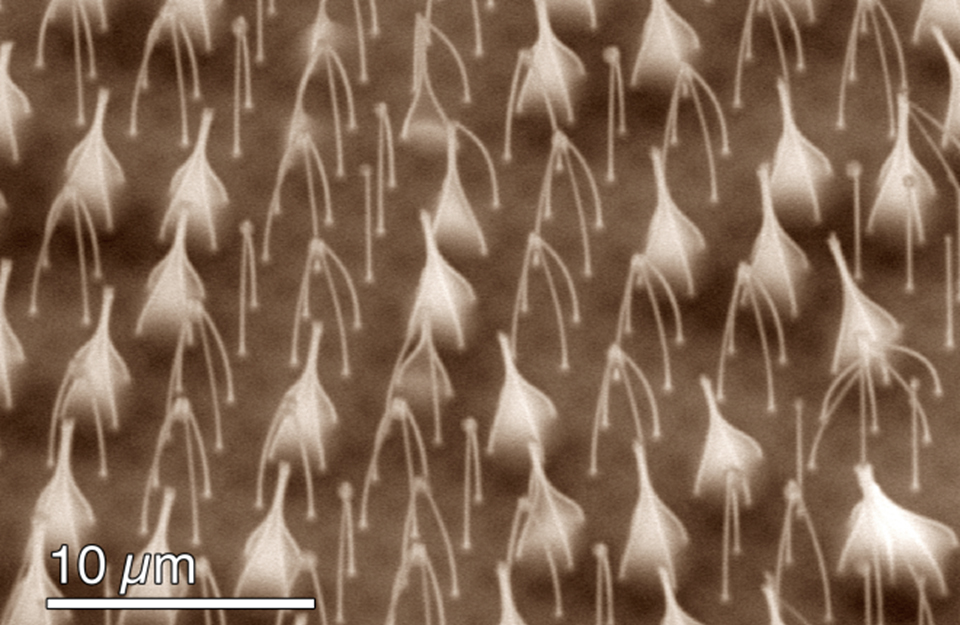
This fluid dynamics video shows the different interaction mechanisms of coalescence-induced droplet jumping during condensation on a nanostructured superhydrophobic surface. High speed imaging was used to show jumping behavior on superhydrophobic copper oxide and carbon nanotube surfaces. Videos demonstrating multi-jumping droplets, jumping droplet return to the surface, and droplet-droplet electrostatic repulsions were analyzed. Experiments using external electric fields in conjunction with high speed imaging in a custom built experimental chamber were used to show that all coalescence-induced jumping droplets on superhydrophobic surfaces become positively charged upon leaving the surface, which is detailed in the video.
Jumping water droplets improve power-plant efficiency
The efficiency of most industrial plants depends crucially on water vapor condensing on metal plates or condensers, and how easily the condensed water can fall away allowing for more droplets to form. On a typical, flat-plate condenser, water vapor condenses to form a liquid film on the surface, drastically reducing the condenser's ability to collect more water, and ultimately acting as a barrier to heat transfer.
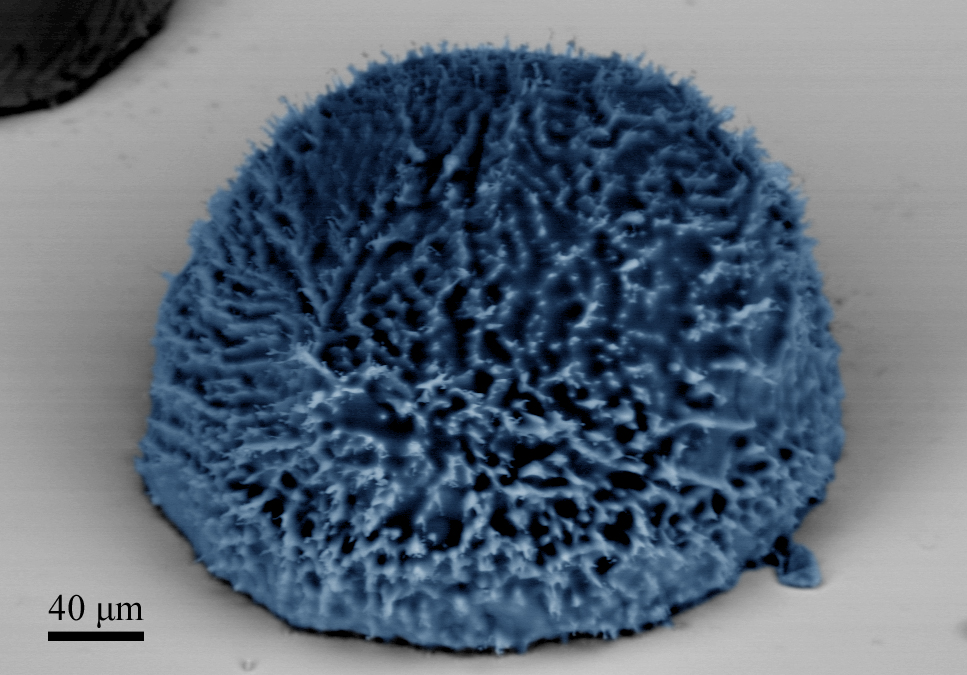
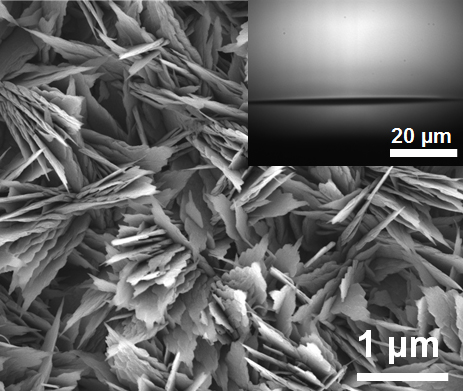
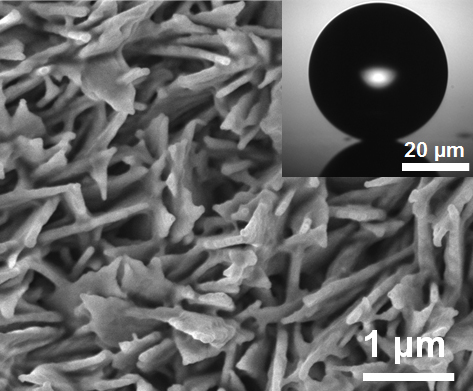
By creating hydrophobic surfaces, either through chemical treatment or through surface patterning, researchers have been able to prevent this problem by encouraging water droplets to form and fall away. Now, we have taken this process a step further by making surfaces that are patterned at multiple scales.
The energy, released as tiny droplets of water that merge to form larger ones, is enough to propel the droplets upward from the surface. The removal of droplets doesn't depend solely on gravity — droplets don't just fall from the surface — they actually JUMP away from it.